Chapter 8: Discussion
The between-family data in this study are consistent with previous MRI studies in showing that there is a significant correlation between brain size and at least some basic cognitive dimensions. It should be made clear that the size of these association is too small to be of any practical importance for, e.g., estimating individuals' IQ scores from their brain size (even between families, less than 22 percent of the variance in the first principal component of the cognitive tests is explained by variance in brain size in this study). What is unique about the present finding is that associations between brain size and cognitive ability do not appear within families. That is, siblings with larger brain sizes do not tend to do better on tests of general cognitive ability.
This study also addressed three other potentially important behavioral dimensions for the first time: degree of sociality, throwing accuracy, and the rate of physical maturation. Sociality showed some evidence of an association with neuroanatomical volume measurements between families, but no solid evidence of an association within families. Throwing accuracy and rate of physical maturation, as measured in this study, were not correlated either between or within families.
There are two important questions raised by these results: 1) how can between-family correlations exist if within-family correlations are absent, and 2) what does this imply about hominid brain size evolution? In addition, the findings in this study have implications for attempts to explain sex differences in brain size. This study provided data relevant to the question of whether grey-white tissue differentiation is related to cognitive function. Lastly, the linguistic tasks devised for this study may have important implications for future research.
8.1 Explanations for Lack of Within-Family Correlations
As discussed in chapter 5, there are two explanations in the literature for how correlations could exist between families but not within families: 1) between-families environmental effects which influence both variables independently, and 2) non-random associations of genetic influences on both variables through some form of cross-assortative mating. There are other possibilities, however, involving within-family environmental effects. In particular, there is evidence for some form of birth-order and/or sibling rivalry effect in the present data set, and further, that this might mask a true genetic correlation between the brain anatomy and behavioral variables measured in this study.
8.1.1 Between-Family Environmental Effects
One explanation involving environmental influences is that the between family correlation is not genetically mediated, but instead is the result of major environmental influences that occur only (or mostly) between families. The most obvious such influence, as pointed out in chapter 5, would be income, or some other aspect of socio-economic status (SES). However, if SES is the actual, underlying cause of the correlation between brain size and behavior, then it would have to be relatively highly correlated with both brain anatomy and the behavioral measures. In order to completely explain the correlation of r= 0.46 between brain volume and the 1st principal component, SES would have to correlate at least r= 0.67 with each of them. However, it is possible that SES explains some portion of the correlation, and since SES information was obtained from these subjects, we can directly assess this hypothesis. SES in this sample was estimated from the following information:
1) Mother's years in school
2) Father's years in school
3) Socio-economic status rating of parents job (during first two years that the subject was in high-school). The family's SES job rating was taken to be that of the parent with the job with the highest rating. The rating was done using a standard SES index (Stevens and Cho 1987).
4) The Home Index of status items. This is a questionnaire about items and aspects of the subject's home environment which are correlated with SES, e.g., whether or not the family owns their own house, has servants, regularly goes on vacations, has more than 500 books, etc. Answers to these sorts of questions are likely more reliable indicators of SES than simply asking someone their parents' income, if for no other reason than that children are often not privy to that sort of information. The particular questions asked were adapted and updated from Gough (1971), and can be found in appendix B.
Each of these four indicators of SES were turned into standard scores and then averaged to yield one composite.
When we look at how this SES measure relates to the other variables in question (table 8.1), we find that it does indeed correlate significantly with the first principal component (r= 0.56) but essentially zero with brain volume (r= 0.05), and non-significantly with the other anatomical variables.[1]
Table 8.1: Between family correlations of SES with neuroanatomic variables1
1st PC BRAINVOL PROSVOL GREYVOL WHITEVOL MMMVOL EFFECT SIZE
SES .56** .05 .02 .06 -.02 .16 .16
SCHOOL-S .24 -.13 -.14 -.31 .09 -.02 .46**
SCHOOL-M .40* .02 .00 .02 -.03 .15 .08
SCHOOL-F .52** .11 .08 .10 .01 .22 .05
* p<.05, ** p<.01
1All variables corrected for age. 1st PC was also corrected for SIMPLE-RT.
Clearly, SES cannot be causing an association between brain size and intelligence if it does not correlate with brain size. If we focus on variability in the first principal component, we see that (in this sample at least) brain size and SES both explain significant, but independent, portions of this variability. If we believe intelligence to be a major cause of SES differences (e.g., Herrnstein and Murray 1994), then these data suggest that even if brain size is causally influencing intelligence, it isn't causing variability that is relevant to SES differences. Conversely, if we believe SES is causing intelligence differences, then we have to believe that the differences SES causes are almost entirely unrelated to the differences associated with brain size. Of course, there might be other variables out there that explain some portion of the correlation between brain size and cognitive test performance, but they would obviously have to be considerably different from (and essentially uncorrelated with) SES.
8.1.2 Cross-Assortative Mating
Another possible explanation for the lack of a within-family association is that the between-family association is due to some form of cross-assortative mating for brain size and general cognitive ability. For this hypothesis to work, there must be a correlation between one parent's brain size and the other parent's cognitive test scores, and the heritability of both variables must be greater than zero. In this model, genes that influence brain size are physically independent of the genes that influence general cognitive ability, but the two sets of genes come to be associated with each other (in individuals) simply because of mating patterns.[2] Jensen (1990) favors this explanation for the lack of a height/IQ association within families (discussed in chapter 5), because it has been shown that, e.g., taller women from a given socio-economic category tend to marry men of higher socio-economic standing (Tanner 1969). Socio-economic standing, in turn, is correlated with IQ (Herrnstein and Murray 1994). There also appears to be an association between height and socio-economic status independent of IQ (Jensen 1990). Thus, there is clear circumstantial evidence that cross-assortative mating is mediating the height/IQ relationship.
There is no direct evidence that cross-assortative mating is the explanation for between-family correlation of brain size and general cognitive ability. It is known, however, that there is significant assortative mating for IQ. Jensen (1978) reports an N-weighted mean correlation for IQ among married pairs of r= 0.42 across a large number of independent studies.[3] At the same time, low but positive correlations have been reported for various physical measurements of the body, including the head. The eight studies of assortative mating for head circumference listed by Spuhler (1968) range from r= -0.02 to 0.24 (N-weighted mean r= 0.09), with similar findings for head breadth and head length.
On the other hand, it is difficult to see how cross-assortative mating would work at a conscious level. One possibility might involve sexual selection for neotenous features, i.e., characteristics of earlier developmental periods, such as a relatively large head in relation to the size of the face. Jones (1995) reports a number of studies indicating that different neotenous features are judged to be more attractive. In one study, pictures of individuals that differed in rated attractiveness were transformed by either slightly expanding the lower face while shrinking the upper head ("positive cardioidal strain"), or vice-versa ("negative cardioidal strain"). The pictures with positive cardioidal strain where consistently found less attractive than untransformed pictures. Female raters, but not male raters, found faces subjected to negative cardioidal strain to be more attractive than untransformed faces. However, the extent to which this preference might be translated into cross-assortative mating between brain size and cognitive test performance is not known.
It should be noted that even if a correlation between brain size and cognitive test performance is only found between families, it is at least theoretically possible for size of the upper cranium to become a desired mating characteristic. Prospective mating partners could still possibly use it as a (very) rough guide to the likelihood of future success, even if it is not directly genetically correlated. This is because the correlation would then be a marker of the well-being of the parents, which in turn is probably not unrelated to reproductive success. Exactly how strong this effect might be has yet to be shown, but it would appear possible. It of course begs the question of how the two variables became correlated in the first place.
8.1.3 Within-Family Environmental Effects
In general, behavioral genetic studies show that a significant portion of the environmental influences on cognitive traits occurs within-families. That is, much of the non-genetic influence tends to make siblings different from one another (as distinct from non-genetic influences such as SES which tend to make families different from one another without differentially affecting siblings within families). For example, Rushton and Osborne (1995) showed that 42% to 52% of the variance in estimated cranial capacity was attributable to within-family environmental influences, while only 6% to 20% was attributable to between-family influences. Similar findings have been found in several twin studies of genetic and environmental influences on personality traits (Bouchard 1994, Rowe 1994), though most of the environmental variance in general cognitive ability (IQ or g) appears to be attributable to between-family influences (Plomin et al. 1990). Because of the relatively restricted range of cognitive ability in the present study, as evidenced by the fact that they had completed three years of college on average, it is likely that the within-family environmental variance accounts for a relatively larger portion of the total non-genetic variance in this sample. This would tend to magnify any non-genetic effects and would contribute to (but probably not completely explain) the lack of within-family associations between neuroanatomy and behavior.
It is at least theoretically possible that there are opposite environmental effects within families on the two variables. Under this model, brain size and cognitive ability are indeed intrinsically linked (that is, genes influencing brain volume directly influence cognitive performance as well), but this causal association is overridden within families by strong (within-family) environmental effects influencing the two variables in opposite directions. An environmental influence within families which tends to increase brain size would have to, at the same time, tend to decrease cognitive performance, and vice-versa. The magnitude of these effects would have to be similar to the magnitude of the between family correlation, so as to negate the association within families. Furthermore, such an environmental influence would have to occur solely within families: if it also operated between families there would be no between-family association to begin with. This model, while not impossible a priori , would not seem to be likely. Though it is testable, I am not aware of any suggestions along these lines in the literature, and apparently no such influences have been proposed (let alone tested).
However, there is evidence in the present study that some form of sibling competition or birth-order effect is affecting within-family associations, which may have the effect of masking any genetic correlation between brain size and behavior that might actually exist. To begin with, the correlation between BRAINVOL and 1st PC is significant if we look only among younger sibs from different families (r=0.34, p<0.05), whereas if we look only at older sibs, the correlation is not significant (r=0.20, NS). Figure 8.1 shows the regressions for older and younger siblings separately. For these calculations the age-corrections and 1st PC scores must be calculated from the overall data set of all 72 subjects (as individuals).[4] Second, at a given age, older siblings tend to do better on the cognitive tests than younger siblings. This can been seen most clearly by comparing the intercepts for the
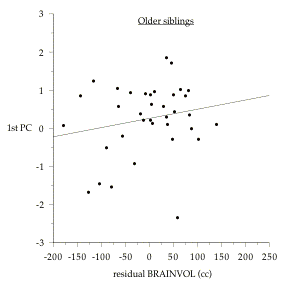

Figure 8.1: Comparison of older and younger sibling relationships between BRAINVOL and 1st PC. Both variables are corrected for age and SIMPLE-RT, using all 72 subjects as individual cases. Older sibling regression: 1st PC = .265 + .002(residual BRAINVOL cc); r= 0.20 (NS). Younger sibling regression: 1st PC = -.27 + .004 (residual BRAINVOL cc); r= 0.34 (p<.05).
regressions of BRAINVOL on 1st PC for the younger and older sibling subsets. Since the BRAINVOL values in this graph are residuals after correcting for age and SIMPLE-RT, the intercepts represent the average 1st PC score at the mean BRAINVOL of all individuals. For older siblings, the intercept is +0.265, whereas for younger siblings it is -0.270: a difference of 0.535. Since 1st PC scores are scaled to a mean of zero and an SD of 1, this means that older siblings, on average, score a little over half a standard deviation higher than younger siblings, even after controlling for age (p<0.03, unpaired t-value=2.26, df=70). On an IQ scale this would translate to a difference of just under 8 points. Furthermore, the slope of the regression line is shallower for older siblings (in line with the smaller correlation between BRAINVOL and 1st PC), which means that the average difference between older and younger siblings tends to increase as average BRAINVOL decreases. In fact, among individuals with smaller than average brains, older sibs have significantly higher 1st PC scores than younger sibs (mean unpaired difference = 0.648, t-value=1.846, p<.04, one-tailed).
Thus, older sibs tend to do better on cognitive tests regardless of their brain size, unlike younger sibs, and (as a consequence) the difference is most pronounced among individuals with lower than average brain sizes. This is consistent with some form of sibling competition, most apparent among older siblings, which causes them to work harder academically than they might otherwise, and thus do better on various cognitive tests. If there actually is a genetic correlation between BRAINVOL and 1st PC, it might be masked by this within-family environmental effect.
The strongest evidence that this is actually occurring in this sample comes from a comparison of the relationship between BRAINVOL and 1st PC among sibling pairs that differ the most in age. In this sample, individuals from families in which siblings are more than four years apart show a statistically significant correlation between brain size and 1st PC (r=0.65,p<0.023, N=12), whereas those closer in age do not (r=0.15, p<0.254, N=60). Within-families, the correlations between BRAINVOL and the cognitive variables (corrected for age and SIMPLE-RT) are higher on average by about 0.24 among sib pairs that differ in age by more than 4 years, as compared to the same correlations for sib pairs differing by less than 4 years (compare tables 8.2 and 8.3). Note that the correlations for the sib pairs that differ by less than four years are essentially the same as those for the 36 pairs as a whole. While several of the correlations for the pairs that are more than four years apart are quite substantial, they fail to reach statistical significance because of the small sample size (N=6). However, the fact that eight of 11 cognitive tests show higher correlations with BRAINVOL in these sibling pairs (i.e., those that differ by more than four years) is consistent with the hypothesis that a birth order effect is swamping the overall within-family correlations.
To what extent are these results explained by the fact that siblings farthest in age are more likely to experience different family environments? Note that sibling pairs in this study were specifically selected to be as close in age as possible, in an attempt to equalize within-family environments. Over the years, parental income and socio-economic status may change. In this study, the SES variables showed very slight (non-significant) differences favoring older siblings. The correlation between sibling age difference and reported family SES information from the subject's high-school years was only r=.11 (NS). Thus, SES differences could not have played a major role in causing these age-related effects (a fact which is confirmed by controlling for SES differences in the variables, which has no effect on the conclusions).
Table 8.2: Within-family
correlations of cognitive to neuroanatomic variables for sibling pairs
that differ by less than four years in age (N=30)1
BRAINVOL PROSVOL GREYVOL WHITEVOL MMMVOL EFFECT SIZE
1st PC -.07 -.05 -.02 -.04 -.15 .00
RAVEN -.09 -.08 .10 -.28 -.13 -.38*
VOCAB .04 .03 .08 -.09 .04 -.17
MRT-SPEED -.29 -.27 -.49** .17 -.23 .51**
MRT-N -.07 -.07 .03 -.13 -.06 -.19
STROOP .10 .12 -.20 .43* -.01 .54**
TRAILS -.15 -.12 -.09 -.02 -.23 .00
VERBALFL -.10 -.09 .10 -.24 -.13 -.26
WCST-PERS .06 -.02 -.04 -.01 .41* -.18
OBJECT-ID -.11 -.11 -.10 -.02 -.07 .12
SENTENCE-VERIF .19 .17 .12 .12 .22 .01
SYNTAX .24 .25 .12 .25 .10 .29
average2 -.02 -.02 -.03 .02 -.01 .03
* p<.05, ** p<.01, 1All variables corrected for age and Simple-RT. 2Excluding 1st PC
Table 8.3: Within-family correlations of cognitive to neuroanatomic variables for sibling pairs that differ by more than four years in age (N=6)1
BRAINVOL PROSVOL GREYVOL WHITEVOL MMMVOL EFFECT SIZE
1st PC .18 .15 .33 -.21 .29 -.27
RAVEN .09 .15 -.15 .36 -.60 .60
VOCAB .77 .76 .72 .26 .05 -.13
MRT-SPEED -.27 -.21 -.48 .41 -.57 .58
MRT-N .82 .79 .85 .25 .27 -.47
STROOP -.11 -.08 -.25 .40 -.31 .36
TRAILS .24 .17 .52 -.34 .70 -.73
VERBALFL -.33 -.36 -.08 -.58 .34 -.22
WCST-PERS .43 .46 .24 .41 -.31 -.01
OBJECT-ID -.23 -.26 -.10 -.44 .22 -.11
SENTENCE-VERIF .53 .48 .70 -.24 .43 -.64
SYNTAX .50 .46 .57 .07 .33 -.25
average2 .22 .21 .23 .05 .05 -.09
* p<.05, ** p<.01, 1All variables corrected for age and Simple-RT. 2Excluding 1st PC
For comparison with analyses in the previous chapter, the mean within-family differences for the various cognitive variables in this study, split into groups of pairs differing by less than years as compared to those differing by more than four years, are included in tables 8.4-8.6. As with tables 7.21-7.26, these are calculated by subtracting the score for the sibling with a smaller BRAINVOL from the scores for their larger-BRAINVOL sibling. If the behavioral measure has some association with BRAINVOL, these values will be positive. These values retain their original units, so that the t-values should be used to compare different behavioral measures. Among the sibling pairs more than four years apart in age, only one cognitive test (MRT-N) showed statistically significantly higher scores (using paired t-tests, one-tailed) in siblings with a larger BRAINVOL (table 8.4). However, eight of the 11 tests at least showed positive t-values (indicating better performance in the sibling with a larger BRAINVOL). Only six of these were higher than the corresponding values for the sibling pairs differing by less than four years of age. Table 8.5 shows the same values for 1st PC, as well as the other cognitive tests after removing variance associated with the 1st PC. These data show that 1st PC among sibling pairs more than four years apart averages 0.22 standard deviations larger in the siblings with larger BRAINVOL, but this is not statistically significant. MRT-N is still significantly larger in siblings with a larger BRAINVOL even after controlling for 1st PC. With the exception of MRT-N and VOCAB, controlling for 1st PC results in lower t-values for the siblings who differ by more than four years in age. By contrast, none of the within-family differences for sibling pairs closer in age than four years reached statistical significance, despite substantially larger sample sizes and larger BRAINVOL differences. Correcting for within-family age differences, siblings who differ
Table 8.4: Average within-family differences for the cognitive variables grouped by within-family age differences
Mean Difference
Variable1 Sibling Age Difference2 N on Variable3 SD t-Value4 Probability5
VOCAB less than 4 years 30 .59 5.94 .55 NS
more than 4 years 6 2.25 6.23 .88 NS
RAVEN less than 4 years 30 .74 5.08 .80 NS
more than 4 years 6 -.08 2.68 -.07 NS
MRT-SPEED less than 4 years 30 -.10 .45 -1.23 NS
more than 4 years 6 .01 .43 .05 NS
MRT-N less than 4 years 30 -.02 4.31 -.02 NS
more than 4 years 6 2.65 1.80 3.61 p<.01
STROOP less than 4 years 30 1.08 10.45 .57 NS
more than 4 years 6 .37 9.82 .09 NS
TRAILS less than 4 years 30 -1.54 10.26 -.82 NS
more than 4 years 6 4.55 8.14 1.37 NS
VERBALFL less than 4 years 30 .41 12.09 .19 NS
more than 4 years 6 -2.33 14.65 -.39 NS
WCST-PERS less than 4 years 30 1.52 15.92 .52 NS
more than 4 years 6 5.08 12.65 .98 NS
OBJECT-ID less than 4 years 30 -30.42 181.96 -.92 NS
more than 4 years 6 -26.55 131.53 -.49 NS
SENTENCE- less than 4 years 30 140.73 955.24 .81 NS
VERIF more than 4 years 6 356.62 570.20 1.53 p<.10
SYNTAX less than 4 years 30 228.92 989.72 1.27 NS
more than 4 years 6 210.92 662.70 .78 NS
1Corrected for age and SIMPLE-RT
2The sibling pairs of less than four years apart had a mean age difference of 2.28 years (SD=0.86), while the sibling pairs of more than four years apart had a mean age difference of 4.78 years (SD=0.495). These groups also differed in mean BRAINVOL differences (corrected for within-family age differences): less than four years: 70.5 cc (SD=60.8); more than four years: 38.7 cc (SD=33.5).
3Mean of (larger-brained sister's score) - (smaller brained sister's score) for each variable
4(Mean Difference)/
5One-tailed
Table 8.5: Average
within-family differences for the First PC and the other cognitive
variables controlling for First PC, grouped by within-family age
differences
Mean Difference
Variable1 Sibling Age Difference2 N on Variable3 SD t-Value4 Probability5
1st PC less than 4 years 30 .02 1.03 .10 NS
more than 4 years 6 .22 .85 .64 NS
RAVEN less than 4 years 30 .68 3.66 1.02 NS
more than 4 years 6 -.79 2.60 -.75 NS
VOCAB less than 4 years 30 .55 5.52 .55 NS
more than 4 years 6 1.73 5.68 .75 NS
MRT-SPEED less than 4 years 30 -.10 .42 -1.36 NS
more than 4 years 6 -.02 .45 -.13 NS
MRT-N less than 4 years 30 -.05 4.06 -.06 NS
more than 4 years 6 2.31 1.21 4.67 p<.005
STROOP less than 4 years 30 1.01 9.21 .60 NS
more than 4 years 6 -.51 11.36 -.11 NS
TRAILS less than 4 years 30 -1.65 8.27 -1.09 NS
more than 4 years 6 3.21 6.12 1.28 NS
VERBALFL less than 4 years 30 .26 9.07 .16 NS
more than 4 years 6 -4.22 8.44 -1.23 NS
WCST-PERS less than 4 years 30 1.61 15.17 .58 NS
more than 4 years 6 6.27 8.83 1.74 p<.10
OBJECT-ID less than 4 years 30 -32.38 141.40 -1.25 NS
more than 4 years 6 -50.97 83.78 -1.49 NS
SENTENCE- less than 4 years 30 129.69 698.57 1.02 NS
VERIF more than 4 years 6 219.37 516.31 1.04 NS
SYNTAX less than 4 years 30 225.43 982.35 1.26 NS
more than 4 years 6 167.62 538.94 .76 NS
11st PC is independent of age and SIMPLE-RT. Other variables controlled for age, SIMPLE-RT, and 1st PC
2The sibling pairs of less than four years apart had a mean age difference of 2.28 years (SD=0.86), while the sibling pairs of more than four years apart had a mean age difference of 4.78 years (SD=0.495). These groups also differed in mean BRAINVOL differences (corrected for within-family age differences): less than four years: 70.5 cc (SD=60.8); more than four years: 38.7 cc (SD=33.5).
3Mean of (larger-brained sister's score) - (smaller brained sister's score) for each variable
4(Mean Difference)/
5One-tailed
by less than four years in age also differed on average by 70.5 cc in BRAINVOL (SD=60.8), while siblings differing by more than four years had an average BRAINVOL difference of only 38.7 cc (SD=33.5). This small within-family difference in BRAINVOL for the sibling pairs greater than four years apart is probably due to sampling error, which future studies could presumably rectify.
For completeness, table 8.6 presents the same analysis for MENARCHE, THROW, and the sociality measures. Of these, only MENARCHE displayed positive t-values for the sibling pairs differing by more than four years of age, though this was not statistically significant. The sociality measures, in particular, display the opposite pattern than that predicted by a simple sibling competition hypothesis. However, it is likely that, in general, there is a trade-off between academic studiousness and social ability and/or social intelligence. It may be that, in general, younger siblings may be more likely to emphasize their social lives while older siblings tend to emphasize academic achievement. Recall the evidence reviewed in section 7.6 that overall cognitive and sociality measures are slightly negatively correlated within families. Since it is clear that older siblings do better academically, it follows that younger siblings should score slightly higher, on average, on sociality measures.
Another characteristic of the data that is clearly consistent with the sibling competition hypothesis is that within-family differences in cognitive test scores are negatively related to within-family differences in age for most of the cognitive variables: that is, sibs who differ the most in age have a slight tendency to be the least different in cognitive test performance (see figure 8.2 and table 8.7). This is highly surprising, especially for tests such as VOCAB, for which it would be easy to assume a priori that as the sibling age
Table 8.6: Average within-family differences for MENARCHE, THROW, and sociality variables grouped by within-family age differences
Mean Difference
Variable1 Sibling Age Difference2 N on Variable3 SD t-Value4 Probability5
MENARCHE less than 4 years 30 -.19 1.34 -.78 NS
more than 4 years 5 .31 .58 1.21 NS
THROW less than 4 years 30 -.60 4.39 -.75 NS
more than 4 years 6 -.35 3.89 -.22 NS
Sociality measures:
ADDRESS BOOK less than 4 years 30 5.75 51.47 .61 NS
TOTAL more than 4 years 6 -28.51 32.13 -2.17 NS
ADDRESS BOOK less than 4 years 30 1.75 6.52 1.47 p<.10
RELATIVES more than 4 years 6 -4.89 6.51 -1.84 NS
PEOPLE SEEN less than 4 years 30 1.62 15.98 .56 NS
SOCIALLY more than 4 years 6 -7.02 16.47 -1.04 NS
PEOPLE TALKED less than 4 years 30 3.25 42.68 .42 NS
TO more than 4 years 6 -38.11 70.45 -1.33 NS
SELF RATING less than 4 years 30 .04 1.36 .15 NS
more than 4 years 6 -.91 .84 -2.66 NS
RATING BY less than 4 years 30 -.42 1.32 -1.73 NS
SIBLING more than 4 years 6 -.33 1.78 -.45 NS
SOCIALITY 1st PC6 less than 4 years 30 .19 .87 1.21 NS
more than 4 years 6 -.90 1.18 -1.86 NS
1Corrected for age. THROW is also corrected for SIMPLE-RT
2The sibling pairs of less than four years apart had a mean age difference of 2.28 years (SD=0.86), while the sibling pairs of more than four years apart had a mean age difference of 4.78 years (SD=0.495). These groups also differed in mean BRAINVOL differences (corrected for within-family age differences): less than four years: 70.5 cc (SD=60.8); more than four years: 38.7 cc (SD=33.5).
3Mean of (larger-brained sister's score) - (smaller brained sister's score) for each variable
4(Mean Difference)/
5One-tailed
6Based on first four sociality measures only
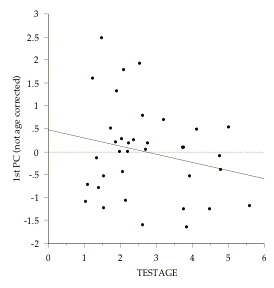
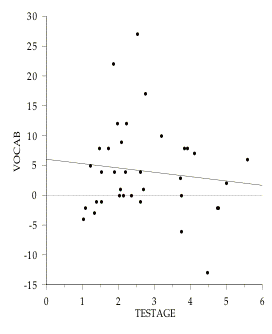
Figure 8.2: Examples of the negative relationhip between age differences and cognitive test score differences within-families. Each point represents a family pair, in which the variables are calculated as (older sibling) minus (younger sibling). The regression lines are: 1st PC (not age corrected) = .484 - .179 (TESTAGE); r2 = .049 (NS); VOCAB = 6.106 - .75 (TESTAGE),r2 = .015 (NS). For eight of the 12 cognitive measures used in this study older siblings do better than younger siblings, and further, this tendency is most pronounced for sibling pairs that are closest in age.
Table 8.7: Within-Family Correlations Between Cognitive Tests and Age at Testing
Variable Age at testing
1st PC -.22
RAVEN -.16
VOCAB -.12
MRT-SPEED -.13
MRT-N -.16
STROOP .06
TRAILS -.23
VERBALFL -.01
WCST-PERS .22
SIMPLE-RT .20
OBJECT-ID -.33*
SENTENCE-VERIF -.04
SYNTAX .18
*p<.05
difference increases, the difference in VOCAB should also increase because the older sibling would have had increasingly greater exposure to vocabulary. Nevertheless, as indicated in table 8.7, for 8 of 11 cognitive tests, as well as the 1st PC of these tests, the slope of a simple regression of age difference on cognitive variable is negative (these tests were corrected for SIMPLE-RT only; correcting for age also would defeat the purpose of this particular analysis). While only one of these negative correlations is statistically significant, the pattern is clear. This would appear to be additional evidence for some sort of sibling/birth order interaction, and is consistent with hypothesis of sibling competition and/or rivalry exhibited most strongly among siblings closest in age.
Another corollary of this hypothesis would be that in families where the younger sib has a larger brain volume, the younger sib should tend to have lower 1st PC than expected Ð the comparison here being with older sibs in families where the older sib also has a larger brain volume. That is, older sibs should be more motivated than younger sibs to do well academically, and take advantage of any benefits brain volume might provide in this realm. This is true for the data in this study, but the difference is far from statistical significance. In families in which the older sibling has a larger BRAINVOL, older siblings score only 0.025 standard deviations higher on average than their younger siblings. By contrast, in families in which the younger sibling has a larger BRAINVOL, the younger sibling averages 0.022 standard deviations lower than their older (and smaller brained) sibling. These values, while in the right direction, could easily be explained by chance (t-value of the difference between these two values is only 0.14, df=34, p=0.44, one-tailed).
If sibling competition is affecting the data in such a way as to cause older siblings to do even better than expected on cognitive tests, we might expect this to cause older siblings to tend to do better regardless of their brain volume. However, in situations where the older sibling has a larger brain but does not score as high on cognitive tests, it might be the case that the younger sibling tends to be exceptionally proficient on these tests. Older siblings, faced with significantly smarter younger siblings, would be less likely to compete academically, thus resulting in lower cognitive test scores than would be the case for younger siblings faced with the same situation. The data in this study are consistent with this idea. Older siblings with larger BRAINVOL and lower 1st PC are relatively poorer on 1st PC than is the case for younger siblings with the same characteristics (that is, who also have larger BRAINVOL and lower 1st PC than their siblings). On average, older siblings with larger BRAINVOL and lower 1st PC scores are 0.85 standard deviations lower on 1st PC than their (younger) siblings. Conversely, younger siblings with larger BRAINVOL and lower 1st PC scores average only 0.53 standard deviations lower on 1st PC than their (older) siblings. The difference between these values just misses statistical significance (t-value =1.58, df=19, p=0.065, one-tailed).
Thus, these data are broadly consistent with the hypothesis that some form of sibling competition/interaction is occurring, and further that this within-family environmental influence could be obscuring a true genetic correlation between brain anatomy and behavior. This would be an important question to address in future studies.
8.2 Implications for Hominid Brain Evolution
The results of this study raise several important issues regarding human neurocognitive evolution. First, we must keep in mind that the range of cognitive dimensions studied in the present research, though broader than any other study to date, does not by any means exhaust all possibilities. As was discussed in earlier chapters, it is highly likely that some behavioral variables are meaningfully associated, in an evolutionary sense at least, with neuroanatomical volume measurements. Second, the size of the associations needed to explain the evolution of the human brain may not have to be very large at all, such that small correlations (requiring much larger sample sizes to show statistical significance) might be all that can be expected. Third, if selection has been operating strongly on brain and behavior, additive genetic variance would, at least theoretically, be reduced. This implies that these features should show relatively higher degrees of environmental influence (in proportion to their overall phenotypic variability). These heightened environmental influences would tend to mask true underlying genetic associations. Important clues might therefore be gleaned from the small but positive associations that were found within families in this study. It is important to remember that the goal of the present study was exploratory in nature: we are interested in what cognitive features might be related to brain size. It is therefore legitimate and important to highlight cognitive tests which show the largest positive correlations within families, while always keeping in mind that the correlations are generally not statistically significant given the sample size.
8.2.1 Other Cognitive Dimensions of Interest
The lack of association between the first principal component and brain volume measures within families, even if this truly indicated that no genetic correlation existed, does not mean that all possible cognitive dimensions are unrelated to brain size. It does suggest that the causal association implied by previous studies will need to be re-examined. Any single study will obviously be limited by practical considerations in the number of different cognitive dimensions that can be assessed, so it is entirely possible that there are other dimensions not measured in this study that are in fact more closely associated with brain size.
One of the most interesting possibilities involves further dimensions of social competence. As was pointed out in chapter 3, it is known that various dimensions of brain size are strongly correlated across primate species with mean group size (Dunbar 1992, 1995; Sawaguchi 1988, 1990; Sawaguchi and Kudo 1990). Measuring social competence in any species is not an easy thing to do, and it is quite possible that the dimensions of sociality addressed (and the measures of social ability used) in this study are not optimal comparisons with the cross-primate data. Future research on this question might profitably use some sort of multiple ranking system, in which individuals (who interact with each other on a daily basis) rank each other with respect to various sociality dimensions. There are also some paper and pencil sociality questionnaires, and at least one has been claimed delimit a social dimension that is independent of IQ (Marlowe 1986), but it is unclear what kind of validity these kinds of tests have for everyday life.
Another set of cognitive dimensions that were not directly tested in this study involve various measures of memory. Speed of access to long term memory was required for OBJECT-ID, but this task only tapped four words, which does not tax the memory recall system (of course, this test was specifically designed to minimize such effects and instead focus on the speed of retrieval of over-learned material). Memory for serial order, as pointed out in chapter 4, is a feature that shows prefrontal specificity. Given the extent to which the prefrontal cortex has expanded during human evolution (chapter 2) it would seem important to address this in future studies, especially given the general lack of significant correlations between neuroanatomical variables and the typical tests of prefrontal function used by neuropsychologists (WCST-PERS, TRAILS, STROOP, and VERBAL FLUENCY, but see below).
Another dimension of memory which should be investigated in some way involves the breadth, depth, and complexity of memories. As was discussed in chapter 3, memories seem to be "stored" widely across the cortex (and sub-cortical areas). Larger brain volume might have some effect on this system, independent of the question of speed of memory retrieval. A closely related possibility is that brain size simply increases the complexity and range of concepts that an individual can process effectively at a given time. All of this would be consistent with Jerison's (1985) suggestion that brain size variation across species represent differences in the complexity of the mental world created by the brain. Cross-modal association would be a related concept as well, since cross-modal association implies the ability to process vastly different kinds of information into a common form. Two cognitive tests are of note in this regard: SENTENCE-VERIF and SYNTAX, both of which had (along with WCST-PERS to MMMVOL), the highest positive correlations within families with the neuroanatomical volume estimates.[5] At the most basic level, these tests require the subject to do both spatial processing (of the arrangement of objects) and linguistic processing (of the sentences). The subject must then compare the results of these two types of processes to determine whether or not they are identical. It is conceivable that those individuals who do the best on these types of tests are better able to process these features in parallel. Alternatively, one could argue that they are better able to translate the two types of information into a common symbolic form to allow them to be compared.
Note that the complexity of one's mental world would not necessarily be assessed by a simple vocabulary test such as was given to the subjects in this study. A given word might conjure up a much richer set to associations in one individual than another (leaving aside the question of how much of vocabulary is simply a reflection of how much people have been explicitly taught), but this would not necessarily be reflected in the raw scores. For what it is worth, within families VOCAB showed the third highest correlation with BRAINVOL of all the cognitive tests, and showed positive correlations with all the volume measures (though none where significant; see table 7.11). This pattern was even stronger when the variance associated with the first principal component was removed (though the correlations were still not significant; table 7.12).
Also, the complexity of one's mental world would not necessarily be assessed by something like the RAVEN's test, which does not require the subject to do many things in many different cognitive dimensions at once. The TRAILS measure used in this study is at least a simple test of this ability, however, in that it requires the subject to keep two procedures in mind at the same time and alternate back-and-forth between them. The results on this test did not correlate with brain size within-families. One might argue post-hoc that it is too simple and might not be complex enough for normal subjects. The STROOP also deals with cross-modal processing, but of a different sort. In this test, one is measuring how easy it is to suppress cross-modal processing. The subject is instructed to ignore the linguistic input, and focus on the color of the items only. Thus, subjects who do relatively poorly on this test might actually be somewhat better at cross-modal processing. In any case, this may be an important direction for future research.
One correlation that appeared in both between- and within-family comparisons, and was consistently independent of the first principal component, was WCST-PERS to MMMVOL. WCST-PERS is essentially a measure of cognitive flexibility in a sorting test. The correct sorting criteria are changed (without informing the subject) each time the subject demonstrates understanding of a given sorting criterion. WCST-PERS is a measure of how often the subject perseverates on the prior (now incorrect) sorting criteria. The cognitive flexibility this test requires might fits well with the idea of brain size corresponding to increased mental complexity: The more complex one's mental world, the easier one would find alternatives when sorting criteria are changed. However, this test has shown specificity to prefrontal damage or abnormalities (Heaton 1981, Weinberger et al. 1986, Rubin et al. 1991) which makes the correlation to non-cerebral areas, as found in this study, curious. The cerebellum (the largest portion of MMMVOL in this study) does have reciprocal connections with the cerebral cortex (via a loop involving cerebrum-to-pontine nuclei-to-cerebellum and cerebellar nuclei-to-ventrolateral nucleus of the thalamus-to-cerebral cortex; Carpenter and Sutin 1986). This might be of interest to future research, assuming this finding can be replicated.
The lack of association of prefrontal tests with the neuroanatomical variables other than WCST-PERS to MMMVOL deserves comment. As was pointed out in chapter 5, there can be a large gap between the extent of the behavioral problems displayed by a patient with prefrontal damage, on the one hand, and the difficulty this patient shows on standardized tests on the other. The tests used in the present study displayed a good deal of variability across the subjects (see table 6.4), and are widely regarded as showing prefrontal specificity. However, it may be that a large portion of the variability in these tests is not specific to prefrontal function, such that normal variation on these tests is not actually variability in prefrontal function per se. These tests are also fairly simple, and it may be that tests need to be more taxing on prefrontal cortical areas if there is to be any association with overall volume. Further investigation of the range of tasks that induce prefrontal activation is obviously important for understanding why the prefrontal region is so large in humans.
Finally, it should be pointed out that the lack of significant correlation between the neuroanatomical variables and throwing accuracy might simply indicate that the measure used to estimate this is not sufficiently related to the types of throwing that were selected for during human evolution. For example, it might be that throwing at a moving target would have been a better measure to use. In any case, however, simple throwing accuracy at a stationary target, at least in this sample of women, would not appear to be a strong candidate for a behavioral adaptation influencing human brain evolution (at least with respect to gross volume measurements).
8.2.2 How Strong Does the Association Need to Be?
Perhaps g (or any of the behavioral dimensions addressed in this study) actually is genetically correlated with brain size within Homo sapiens, but the correlations are too small to be apparent (or statistically significant) without huge sample sizes. The fact that rate of maturation correlates so strongly with brain size across primates (r= 0.96, Harvey and Clutton-Brock 1985), as pointed out in chapters 3 and 5, but very little if at all among human females, may say a lot about what our expectations should be with respect to within species behavior associations with gross neuroanatomical dimensions. Note that rate of maturation is a variable which is directly comparable across species, and this fact distinguishes it from various cognitive tests which generally cannot be given to other species, and for which there can be no direct comparability across species as a consequence. While it is true that rate of maturation as measured in this study is not perfectly reliable, the lack of a correlation (even between families) should make us consider the possibility that associations with neuroanatomy may vary dramatically between versus within species. We should keep in mind, however, that the association between brain size and maturation holds at a gross level between human sexes: females mature more quickly relative to males, and also have smaller brains on average (even after correcting for body size differences; Ankney 1992).
We must keep in mind that, for long term evolutionary questions, small within-species correlations are not irrelevant. It is known that, at least theoretically, huge changes can occur over evolutionary time in response even to only extremely small selective forces acting on moderately correlated characteristics (Lande 1976). This suggests that huge changes might be possible in response to strong selection on weakly correlated characters. As a direct example of this, Van Valen (1974) calculated that a change of one standard deviation in brain size (~150 gm) would only take about 8,000 generations assuming 1) a genetic correlation of only r= 0.10 between brain size and intelligence (or whatever correlated feature of brain size that was selected for), and 2) that the small empirical associations between IQ and number of offspring in humans (Bajema 1966) was constant through time. If the average generation length was 20 years, it would take less than 1.1 million years to change average brain size from ~450 gm to 1450 gm.[6] This is less than half the time over which the change actually occurred, as judged from the fossil record (chapter 2). It is therefore quite possible that real associations exist, but are so small that we would never reasonably expect to detect them within humans (or any species) given the current cost of MRI. However, this suggestion does leave us with the question of why brain size appears to be associated with various behavioral dimensions in rats (chapter 4), as well as why robust, replicable associations should exist between families in humans.
8.3 Sex Differences
The present study also has relevance to the question of sex differences. The finding that mental rotation speed (MRT-SPEED) does not significantly correlate with brain size (even between families) suggests that the sex difference in brain size (Ankney 1992) cannot be explained by this difference in cognitive performance. It is important to note that mental rotation tests show the largest, most consistent sex difference (Linn and Petersen 1985). Furthermore, while MRT-N is significantly correlated with brain size between families, it is not significantly correlated within families (MRT-N is only weakly correlated with MRT-SPEED, see tables 7.7, 7.8, 7.9, 7.11). Given that the cognitive test showing the largest sex difference does not correlate with brain size within females, it would appear either that sex differences in brain size are not functional with respect to cognitive performance, or that the brain size difference is attributable to some other cogntive dimension (which would presumably display less of a sex difference than mental rotation ability). There are two caveats, however. First, given the evidence that some within-family environmental effects may be masking intrinsic relationships, there may still be a genetic correlation between the two that is masked. Secondly, the brain size association with mental rotation ability does hold when comparing sexes. That is, males score higher on these sorts of tests and also have larger brains than females (as noted above; Ankney 1992).
8.4 Grey-White Matter Contrast and the Structure of the Brain
While questions surrounding the grey-white matter tissue differentiation are not the focus of this study, there are some tentative conclusions that can be drawn from the analysis of these data which may be important for further study. The fact that EFFECT SIZE correlated significantly both between and within families may indicate that individuals with greater WHITEVOL have more white matter pixels at the higher end of the white matter distribution. The white-grey differentiation in MRI is thought to be due primarily to the relative amounts of cholesterol in these tissues (Koenig 1991, see review in Schultz et al. 1992). Cholesterol is a major component of myelin, which in turn makes up half the dry mass of white matter (Morell et al. 1989). Thus, individuals with larger white matter volumes as estimated in this study may have disproportionately more myelin. The fact that individuals with larger grey matter volumes have less grey-white differentiation may indicate that additional grey matter increases the number of myelinated fibers which would be found in grey matter regions. This would have the effect of adding pixels of intermediate value between pure grey and white matter pixels, and these intermediate pixels would tend to be assigned to grey matter regions by the curve fitting method used in this study. This is only one possibility, of course. The extent to which this is the correct interpretation will have to wait for further study.
Behaviorally, EFFECT SIZE did not correlate significantly (either between or within families) with the 1st PC, but EFFECT SIZE did correlate substantially with MRT-SPEED and STROOP within families (the between family correlations for these variables were positive but not significant; see tables 7.5 and 7.7). Schultz et al. (1992) report that T2 image contrast between grey and white matter regions correlated significantly with a battery of subtests from the WAIS intelligence test. Only one of these tests was directly comparable to a test given in the present study: vocabulary. Their vocabulary test correlated r= 0.51 with their estimate of image contrast. By comparison, the vocabulary test in the present study correlated r= -0.08 (both between and within families; see tables 7.5 and 7.7). The Schultz et al. (1992) study included a test called "block design", which is essentially a spatial test, but is not a direct test of mental rotation speed (as is the variable MRT-SPEED used in the present study). However, Schultz et al. (1992) report a correlation of r= 0.41 between block design and T2 image contrast, which is similar to the value reported above for MRT-SPEED. Schultz (1991) reported that overall IQ correlated r= 0.48 with T2 contrast. In the present study, the 1st PC (an estimate of overall IQ) correlated r= 0.24 (NS) between families (the most directly comparable correlation), but only r= 0.06 (NS) within families (tables 7.6 and 7.8). The effects of range restriction and differences in reliability (as discussed in the previous chapter) may partly explain the low within family correlation, but the between family correlation is also non-significant.
The different results found in these two studies may partly be due to methodological differences, however. Image contrast in the Schultz et al. (1992) study was estimated by calculating T2 relaxation times (based on pixel intensities at two different echo times[7]) for pre-defined grey and white matter regions, and then subtracting T2white from T2grey. However, EFFECT SIZE in the present study would appear to be an objective measure of grey-white differentiation as well. This will need to be addressed more fully in future research (data was collected to allow direct comparison with the Schultz et al. 1992 study, but was not analyzed for the present study). Should the findings in the present study hold, it would appear that grey-white differentiation is not significantly associated with overall IQ, though it might with mental rotation ability. If the reason some individuals in this study have larger white matter volumes is that these individuals have more myelin, then the general findings in this study would not be consistent with the hypothesis that variability in degree of myelinization accounts for a significant portion of IQ differences (Miller 1994). WHITEVOL and GREYVOL explain almost equal amounts of 1st PC variability between families, and neither explain much within families. Much work needs to be done, however.
8.5 Possible Physiological Explanations of Brain Size Increases
With respect to the actual genetic, physiological mechanisms by which brain size increases occurred during human evolution, little is known with any confidence. As mentioned briefly in the introduction, one possibility is that point mutations increased the efficiency, speed, reliability, etc. of basic physiological processes that play a key role in synaptic transmission and plasticity. Because both developmental processes and the maintenance of existing synapses in the brain are use-dependent (Purves 1988, Artola and Singer 1994), it is plausible that even small changes in the efficacy of basic physiological pathways involved in synaptic transmission would result in increases in overall neural volume. That is, by making the feedback more effective, fewer neurons will be pruned in the first place and existing connections will spur even more synaptic growth between existing neurons. One such physiological pathway involves N-methyl-D-aspartate (NMDA) receptors, which appear to play a key role in detecting correlations between pre- and post-synaptic activity, thereby facilitating the enhancement and expansion of synaptic connections between neurons (Artola and Singer 1994). NMDA receptors appear to be important for various types of learning and memory (Morris and Davis 1994). These are only preliminary suggestions, of course, but they are intriguing possibilities.
8.6 Linguistic Tasks
One important finding of this study is that there exist individual differences in the efficiency with which different syntactic forms are processed. These differences do not appear to be caused by differences in past linguistic exposure to these syntactic forms (though this was only indirectly tested in this study). This contradicts the supposition by Chomsky and others that no meaningful variation exists in the ability to process the basic rules of language. The findings are congruent, however, with the idea that language evolved through modifications of existing forms, such that individuals would be expected to vary on all linguistic (and cognitive) dimensions. Future studies using tasks similar to the one used in the present study should provide additional data relevant to this question.
The fact that right-branching sentence verification times are closely predicted by a simple extrapolation from OBJECT-ID times, whereas center-embedded sentence verification times are not, may well indicate something about our cognitive processing in general. Clearly, some syntactical forms carry greater processing burdens than others. The interruption of one clause by another is less congruent with our underlying cognition than is the linear stringing of clauses one after another. If the process of thinking involves tracing a path through a particular web of semantic associations, and if language is simply a method of communicating these particular paths, then it makes sense that syntactic forms which cause the paths to be interrupted would be inherently more difficult to process. This model is congruent with what we know about how memories are stored and how words appear to represent webs of associations between different cortical areas, as discussed in chapter 3.
However, why would forms that interrupt these linear paths exist at all? For one thing, the interruptions allow the speaker to place a particular node in the web into more definite context. For example, consider the sentence:
"The square which the circle is above, is beside the triangle."
The middle clause of this sentence ("...which the circle is above...") puts the subject of the sentence into a more definite context, which is presumably a useful thing to do. However, this comes at the expense of having to keep the subject of the sentence immediately in mind so that it may be reconnected with the final (main) clause.
Clearly, these are only preliminary suggestions, but the basic reaction-time findings in this study fit a general model of cognition which assumes that linguistic forms adapted to pre-existing cognitive structures, as opposed to creating entirely new structures de novo during the evolution of language.
8.7 Summary
This study does not demonstrate that brain size and cognitive performance are genetically unrelated, but it does constrain the possible explanations and raises a number of important issues for future research relating to the evolution of brain and behavior. The lack of a within-family correlation for general cognitive ability does not appear to be the result of range-restriction or differential reliabilities of within- vs. between-family calculations, but it also is not explained by SES, which is the most obvious possible explanation for a purely between family environmental cause. There is strong suggestive evidence in the data for within-family environmental effects, perhaps the result of some form of sibling rivalry, and these effects may be an important part of the explanation for the lack of within-family associations. It is also at least theoretically possible that some within-family environmental effects influence neuroanatomy and behavioral ability in opposite ways, though none have been proposed so far. Furthermore, other behavioral variables not measured in this study might prove to be significantly correlated with brain size within families in humans (even in restricted samples such as this), and some variables (such as those dealing with sociality, prefrontal abilities, and throwing) might well be measured better by some other methods than those used in this study. Alternatively, the size of the true genetic correlations might be too small to be detected without large sample sizes within a species. The fact that MENARCHE correlates so strongly between species, but essentially zero in this data set (either between or within families), strongly supports this possibility. Cross-assortative mating remains a possibility, though the mechanism by which this might occur is obscure. We must also keep in mind that none of these explanations are mutually exclusive. In the final analysis, it may well be that all of them have some partial explanatory value: true genetic correlations between brain anatomy and behavior are lower than that indicated by between-family correlations, but significantly greater than zero, at least with respect to evolutionary processes.
Explanations for the sex difference in brain size are not easily explained by sex differences in spatial ability, because the within-female associations are too small, though an association still exists if we dichotomize brain size and spatial ability by sex. Finally, significant variation exists at the individual level in syntax processing speed across different syntactical forms, and this variation is not easily explained as a result of the amount of past exposure to these forms. These findings are consistent with a model of language evolution in which pre-existing cognitive structures were co-opted for use by language. Much work remains to be done on this issue.